Our Vision
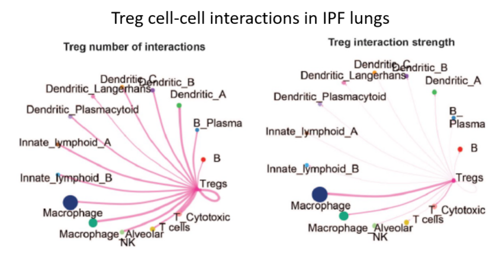
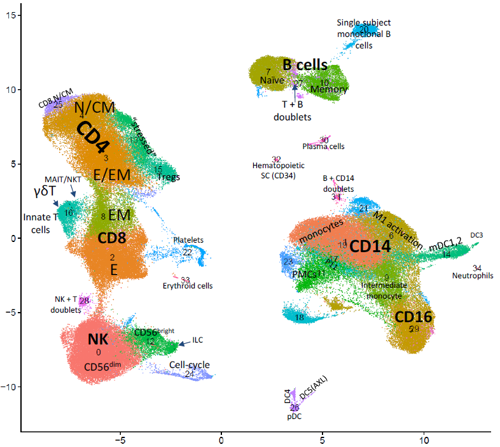
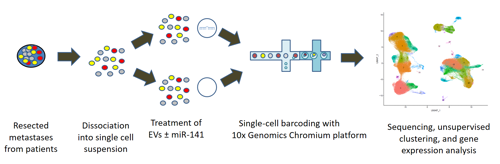
To uncover the basic mechanisms underlying lung fibrosis and develop new treatments - Interstitital lung diseases (ILDs) consist of over 200 different diseases. Many patients, in particular those who are diagnosed with idiopathic pulmonary fibrosis (IPF), develop severe lung scarring (called lung fibrosis) that necessitates lung transplantation for survival, including some who recover from COVID-19. Despite multidisciplinary approaches to diagnose ILD patients, in many cases the exact diagnosis is unclear. Furthermore, current treatment options may delay disease progression but cannot provide cure. In our laboratory, we employ cutting-edge genomic technologies, including single cell RNA sequencing (scRNA-seq), to study ILD patient-derived samples. We believe that by using scRNA-seq, we may come one step closer towards elucidating the biology of these intricate diseases, identify new biomarkers and develop novel treatments for ILD patients. of Regulatory T Cells in Pulmonary Fibrosis (In collaboration with Prof. Yifat Merbl, Weizmann Institute, and Dr. David Hagin, TASMC) Idiopathic pulmonary fibrosis (IPF) is a non-curable, devastating disease. Our recent scRNA-seq profiling of the peripheral blood immune cells in IPF (Unterman et al., AJRCCM, 2024) revealed an outcome-predictive increase in regulatory T cells (Tregs) in progressive disease, as well as evidence for a potential recruitment of Tregs from the blood to the lung. In order to comprehensively characterize the role of Tregs in pulmonary fibrosis, we will perform a multiomics characterization of Tregs using cutting edge transcriptomics and proteomics approaches, complemented by in vitro and in vivo validations of identified mechanisms. Altogether, this project aims to elucidate novel immunological mechanisms implicated in IPF pathobiology that may be used to devise novel immune-related therapeutic approaches in pulmonary fibrosis. fibrotic hypersensitivity pneumonitis (fHP) Among of the most common ILDs are idiopathic lung fibrosis (IPF), chronic hypersensitivity pneumonitis (CHP) and connective tissue-related ILDs (CTD-ILDs). It is important to differentiate between these diagnoses, since CHP and CTD-ILD require immunosuppressive therapies, while IPF is exacerbated by this treatment regime and require anti-fibrotic agents instead. We wish to analyze immune cells, which can be easily extracted from patients blood and bronchoalveolar lavage fluid, by utilizing scRNA-seq. This analysis will facilitate the identification of immunological mechanisms that drive these diseases and allow the development of precision medicine approaches for diagnosis and treatment of extracellular vesicles (EVs) in pancreatic cancer metastasis Pancreatic cancer is among the leading causes of cancer-related mortality, in which omental metastasis is a frequent event that is associated with very poor prognosis. In this study, we will examine the effects of EVs enriched with miR-141 on pancreatic cancer patient-resected metastases, as a potentially novel therapeutic approach. We will leverage on single-cell RNA sequencing in order to examine the intricate tumor-stroma interactions and biological mechanisms that govern the propagation of these metastases, as well as the potential therapeutic effect of EVs. This project is performed in collaboration with Prof. Guy Lahat and Dr. Shelly Loewenstein from the surgical oncology research laboratory. in COVID-19 patients COVID-19 may manifest itself as a mild to moderate disease, or as a severe disease requiring intensive care unit (ICU)-level care that may include mechanical ventilation and extracorporeal membrane oxygenation (ECMO). Evidence suggest that dysregulated inflammation plays an important role in the exacerbation of the disease, which may lead to an increased morbidity and mortality of the disease. Nonetheless, the exact molecular mechanisms accounting for this immune dysregulation, as well as their modulation during different stages of the disease, are not fully characterized. In a joined effort together with five different laboratories in Yale School of Medicine, we performed an in-depth, single-cell multi-omics analysis of immune cells that were derived from progressive and stable COVID-19 patients. This analysis revealed unique cell populations of both the innate and adaptive immune cells, as well as aberrant cell-cell interactions between them, that characterized progressive COVID-19 patients. In addition, unique profiling of T cells and B cells revealed a skewed expansion of specific clones that characterized progressive patients. To conclude, this study revealed a dyssynchrony of the innate and adaptive immune system in progressive COVID-19 patients. From The Press
Highlight Publications
Our Team
Research