Our Vision
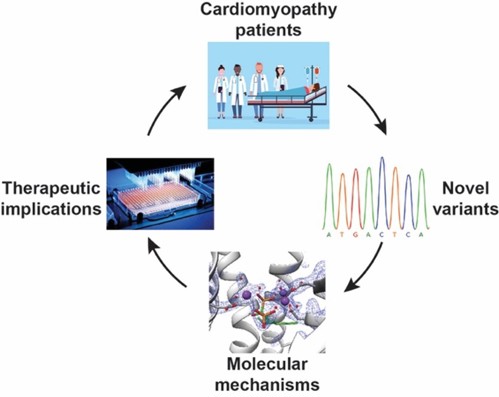
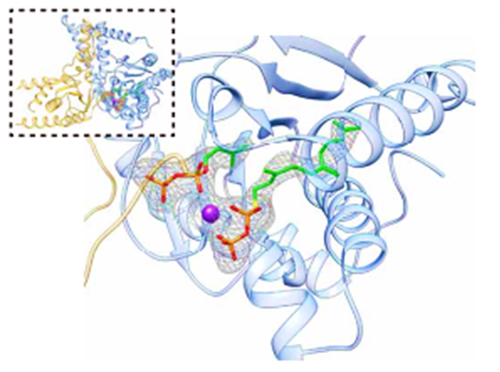
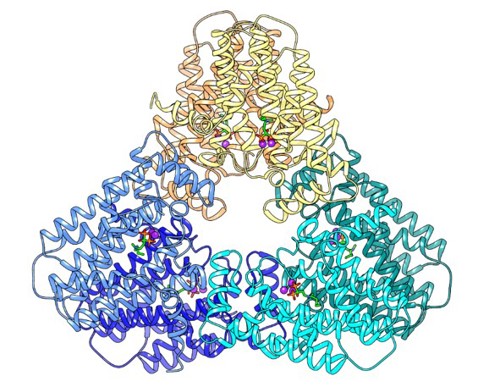
Our lab aims to uncover the molecular mechanisms driving human disease and to translate this understanding into new therapeutic strategies. We use a cutting-edge, multidisciplinary approach that combines computational biology, biochemistry, and pharmacology to study the structure-function relationships of clinically important proteins. Dr. Moshe Giladi, M.D., Ph.D., Lab PI Dr. Saray Tabak, Ph.D., Lab manager Proper heart contraction and adequate cardiac output depend on coordinated activity – from the sarcomere to the tissue level. Inherited cardiomyopathies are a diverse group of heart disorders characterized by structural and functional abnormalities of the myocardium. Genetic diagnosis of inherited cardiomyopathies remains non-trivial due to marked clinical and genetic heterogeneity, with incomplete penetrance and variable expressivity leading to ongoing challenges in the classification of novel variants. In collaboration with the cardiology division at TASMC, we developed a versatile platform for characterizing novel variants identified in our patients and suspected to cause cardiomyopathy. This platform is used to assess the functional and structural impacts of these mutations at the protein level, classify novel variants and explore possible therapeutic interventions. Indeed, we have recently established the pathogenic role of a novel SLC4A3 variant in short QT syndrome and developed a bedside clinical diagnostic test. of DHDDS-related retinitis pigmentosa Protein N-glycosylation is a vital post-translational modification essential for correct protein folding, oligomerization, quality control, intracellular sorting, and trafficking. This process involves the transfer of an oligosaccharide moiety from the lipid carrier dolichol-phosphate (Dol-P) to specific asparagine residues on nascent polypeptides. We investigate the enzymes responsible for the synthesis and processing of dolichols as mutations in these enzymes have been linked to a group of rare but devastating conditions known as congenital disorders of glycosylation (CDG). These disorders manifest with a wide range of clinical symptoms, including blindness, neurodevelopmental defects, and in severe cases, lethal systemic dysfunction. By combining computational and experimental structural biology approaches such as molecular dynamics and X-ray crystallography with biochemical assays and cellular models, we explore how these enzymes function, how they are regulated, and how their dysfunction leads to disease. A major goal of our research is to develop small-molecule therapeutics that can restore or modulate enzyme activity, offering a precision medicine approach for patients with CDG and related disorders. translational protein modifications Post-translational protein modifications (PTM) are crucial for protein folding, function, and cellular localization in every cell in the human body. These modifications were highlighted in cancerous transformation, metastasis, and response to treatments. However, no specific treatments targeting aberrant PTM are currently available for clinical use. By studying key enzymes involved in the synthesis of moieties for PTM, we use a structure-guided approach to develop novel and specific cancer therapies.
By integrating in silico modeling, high-resolution structural analysis, and functional assays, we work to rationally design small-molecule therapeutics targeting proteins involved in inherited disorders and cancer. Our Team
Research
Publications
Structural Biology of Disease Lab
Dr. Moshe Giladi, M.D., Ph.D., Lab PI
A full list of publications can be reached at NIH