Oncology Division Research Laboratory
R&D > Laboratories > Oncology Division Research Laboratory

Our Vision
Cancer is not an isolated process and its development is tightly associated with endocrine and metabolic changes in the body. As a lab sitting next to the Oncology Division, the bench and bedside are constantly intertwined. Therefore, we are not only focused on the patient's tumor, but know that the cancer disease is affected by many other factors. By investigating the complex interactions between the tumor, the received treatments and basic patient's characteristics, we are able to decipher novel endocrine aspects of malignancy and gain new insights into the development of cancer. This in turn uncovers vulnerabilities of the cancer and may aid in the development of novel treatments.

Contact Us
Primary Investigators

Ido Wolf Lab PI
Dept. of Internal Medicine052-7360558 idow@tlvmc.gov.il

Tami Rubinek Lab PI
Dept. of Internal Medicine052-7466151 tamarru@tlvmc.gov.il
General Contact

Keren Marenbakh - Lamin, PHD Lab PI
03 - 6972412 kerenme@tlvmc.gov.il
Address
Sammy Ofer Heart Building
10th floor Room 12

Research
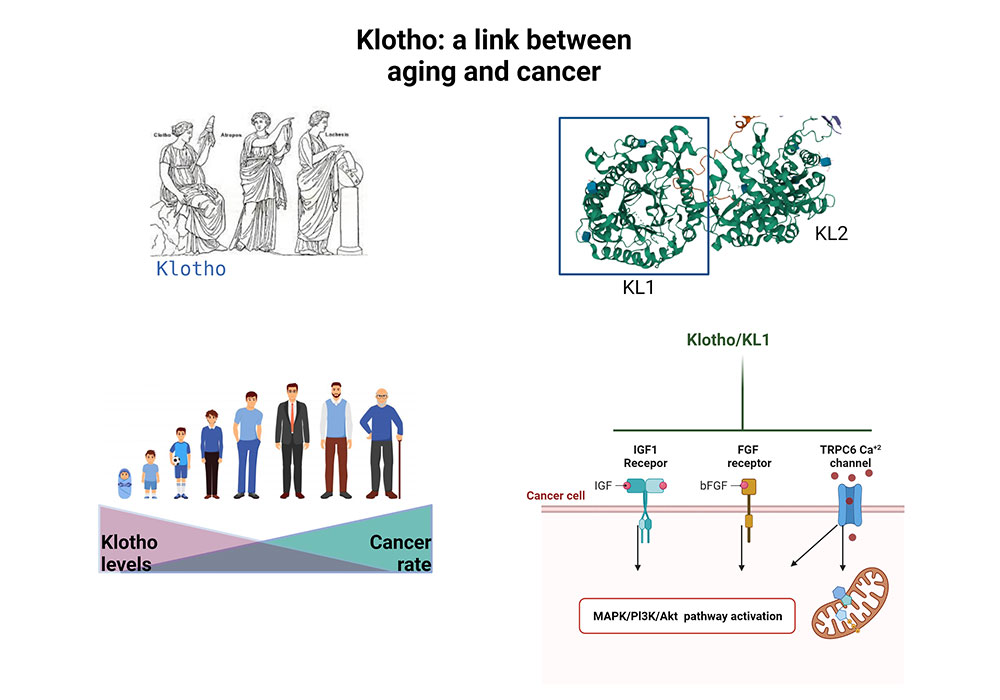
Aging and cancer development are tightly connected. The hormone klotho plays an important role in human aging. Our lab discovered its role as a tumor suppressor gene. It is silenced in cancer tissues and klotho’s re-expression in cancer cells inhibits their growth. Further analysis revealed klotho as a modulator of signaling pathways, especially those that regulate metabolic pathway.
Currently, we are focusing on discovery of its putative receptor and developing novel klotho-based treatments against cancer.
Our lab was the first to discover mutations in ESR1 that confer resistance to hormonal therapies in >40% of patients with metastatic breast cancer. We are trying to find the relationship between mutations and a more aggressive disease as well as finding new treatment strategies.
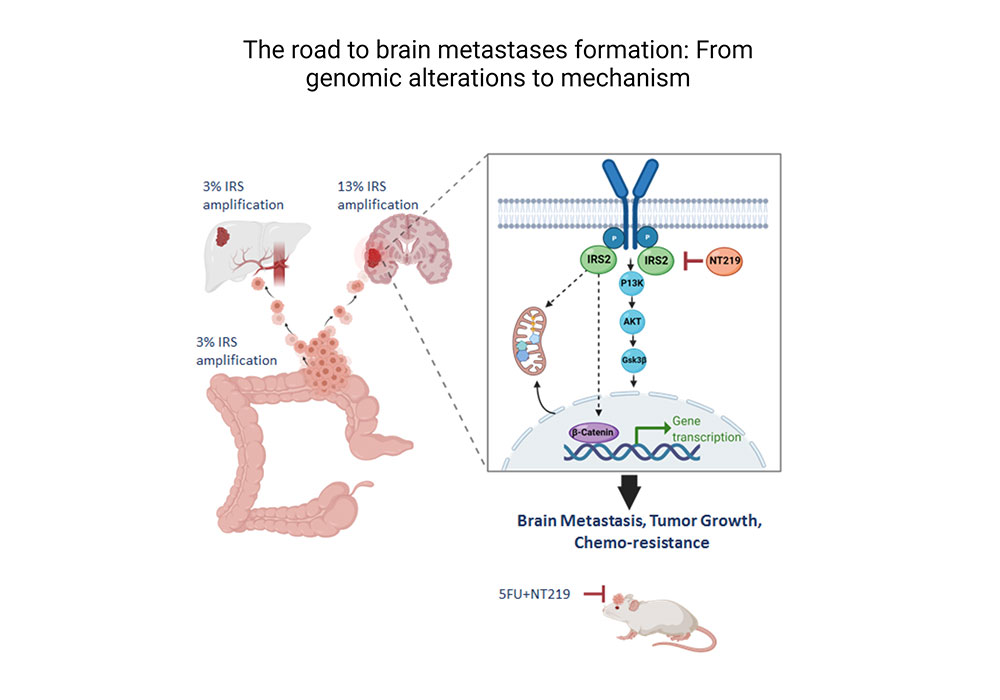
We are tackling the role of specific mutations in mediating homing of cancer cells to specific organs. Using large patients’ databases and advanced bioinformatics we identify genes and pathways that coax homing of cancer cells to specific organs. Specifically, we are focusing on the relationship between the mutations and metabolic pathways.
Gallery









Our Team
Current Staff
Researchers
- Hagai Ligumsky, MD, PhD
- Keren Marenbakh-Lamin, PhD
Research asociate
- Fadi Rizqa, MSc
Students
- Arkadi Hesin, PhD student
- Lotem Zinger, PhD student
- Inbal Greenberg, MD/PhD student
- Shani Journo, PhD student
- Marwa Taya, PhD student
- Marana Abboud, MSc student
- Sivan Fuchs, MSc student
MD thesis
- Shai Bar-Shira
Past Staff
Researchers
- Lilach Abramovitz, PhD
- Ayelet Orbach-Shabtay, PhD
Students
- Smadar Levanon, MSc
- Shiri Shahmoon, PhD
- Tammi Rubinstein, MD, PhD
- Ira Lojkin, PhD
- Riva Shmulevich, PhD
- Inbal Reuveni, MSc student
- Adi Elazar, MSc
Madaei Yesod
- Gil Har Zahav
- Anton Wol
MD thesis
- Shira Hasson, in honor
- Eliya Shachar, in honor
- Tal Eitan, in honor
- Bar Davidov, in honor
- Tomer Boldes, in honor
Current funding





Highlight Publications
Journo S, Goldberg AK, Sokol ES, Zinger L, Pasmanik-Chor M, Sarvin B, Simkin D, Fuchs S, Shlomi T, Wolf I, Rubinek T. Oncogene. 2022 Mar;41(10):1468-1481
Klotho rewires cellular metabolism of breast cancer cells through alteration of calcium shuttling and mitochondrial activity. Shmulevich R, Nissim TB, Wolf I, Merenbakh-Lamin K, Fishman D, Sekler I, Rubinek T.
Oncogene. 2020 Jun;39(24):4636-4649.
Ligand-binding domain-activating mutations of ESR1 rewire cellular metabolism of breast cancer cells. Zinger L, Merenbakh-Lamin K, Klein A, Elazar A, Journo S, Boldes T, Pasmanik-Chor M, Spitzer A, Rubinek T, Wolf I.
Clin Cancer Res. 2019 May 1;25(9):2900-2914.
More Publications >>
Genomic alterations drive brain metastases formation in colorectal cancer: The role of IRS2.
Greenberg I, S. E., Klein Goldberg A, Simkin D, Benhamou M, Bar-Shira S, Raz M, Grossman R, Yeini E, Ofer P, Liang B, Satchi-Fainaro R, Reuveni H, Rubinek T, Wolf I. Biorxiv Biorxiv/2022/495658 (2022).
A Transgenic Model Reveals the Role of Klotho in Pancreatic Cancer Development and Paves the Way for New Klotho-Based Therapy.
Arbel Rubinstein T, Reuveni I, Hesin A, Klein-Goldberg A, Olauson H, Larsson TE, Abraham CR, Zeldich E, Bosch A, Chillón M, Hollander KS, Shabtay-Orbach A, Vainer GW, Wolf I, Rubinek T. Cancers (Basel). 2021 Dec 15;13(24):6297.
R269C variant of ESR1: high prevalence and differential function in a subset of pancreatic cancers. Boldes T, Merenbakh-Lamin K, Journo S, Shachar E, Lipson D, Yeheskel A, Pasmanik-Chor M, Rubinek T, Wolf I.
BMC Cancer. 2020 Jun 8;20(1):531.
Role of Klotho protein in tumor genesis, cancer progression, and prognosis in patients with high-grade glioma. Peshes-Yeloz N, Ungar L, Wohl A, Jacoby E, Fisher T, Leitner M, Nass D, Rubinek T, Wolf I, Cohen ZR.
World Neurosurg. 2019 Oct;130:e324-e332.
Klotho suppresses colorectal cancer through modulation of the unfolded protein response. Arbel Rubinstein T, Shahmoon S, Zigmond E, Etan T, Merenbakh-Lamin K, Pasmanik-Chor M, Har-Zahav G, Barshack I, Vainer GW, Skalka N, Rosin-Arbesfeld R, Varol C, Rubinek T, Wolf I.
Oncogene. 2019 Feb;38(6):794-807.
New somatostatin-drug conjugates for effective targeting pancreatic cancer. Ragozin E, Hesin A, Bazylevich A, Tuchinsky H, Bovina A, Shekhter Zahavi T, Oron-Herman M, Kostenich G, Firer MA, Rubinek T, Wolf I, Luboshits G, Sherman MY, Gellerman G.
Bioorg Med Chem. 2018 Jul 30;26(13):3825-3836.
Klotho response to treatment with growth hormone and the role of IGF-I as a mediator. Rubinek T, Shahmoon S, Shabtay-Orbach A, Ben Ami M, Levy-Shraga Y, Mazor-Aronovitch K, Yeshayahu Y, Doolman R, Hemi R, Kanety H, Wolf I, Modan-Moses D.
Metabolism. 2016 Nov;65(11):1597-1604.
Alteration in serum klotho levels in anorexia nervosa patients. Wolf I, Stein D, Shahmoon S, Ziv SI, Hemi R, Kanety H, Rubinek T, Modan-Moses D.
Clin Nutr. 2016 Aug;35(4):958-62.
Klotho expression in cervical cancer: differential expression in adenocarcinoma and squamous cell carcinoma. Aviel-Ronen S, Rubinek T, Zadok O, Vituri A, Avivi C, Wolf I, Barshack I.
J Clin Pathol. 2016 Jan;69(1):53-7.
Tumor suppressor activity of klotho in breast cancer is revealed by structure-function analysis. Ligumsky H, Rubinek T, Merenbakh-Lamin K, Yeheskel A, Sertchook R, Shahmoon S, Aviel-Ronen S, Wolf I.
Mol Cancer Res. 2015 Oct;13(10):1398-407.
The effect of klotho treatment on atherogenesis, blood pressure, and metabolic parameters in experimental rodent models. Kamari Y, Fingrut O, Shaish A, Almog T, Kandel-Kfir M, Harats D, Rubinek T, Wolf I.
Metab Res. 2016 Mar;48(3):196-200.
Reduced expression and growth inhibitory activity of the aging suppressor klotho in epithelial ovarian cancer. Lojkin I, Rubinek T, Orsulic S, Schwarzmann O, Karlan BY, Bose S, Wolf I.
Cancer Lett. 2015 Jul 1;362(2):149-57.
Association between decreased klotho blood levels and organic growth hormone deficiency in children with growth impairment. Wolf I, Shahmoon S, Ben Ami M, Levy-Shraga Y, Mazor-Aronovitch K, Pinhas-Hamiel O, Yeshayahu Y, Hemi R, Kanety H, Rubinek T, Modan-Moses D.
PLoS One. 2014 Sep 8;9(9):e107174.
The aging suppressor klotho: a potential regulator of growth hormone secretion. Shahmoon S, Rubinfeld H, Wolf I, Cohen ZR, Hadani M, Shimon I, Rubinek T.
Am J Physiol Endocrinol Metab. 2014 Aug 1;307(3):E326-34
Association between variants of 5-hydroxytryptamine receptor 3C (HTR3C) and chemotherapy-induced symptoms in women receiving adjuvant treatment for breast cancer. Pud D, Har-Zahav G, Laitman Y, Rubinek T, Yeheskel A, Ben-Ami S, Kaufman B, Friedman E, Symon Z, Wolf I.
Breast Cancer Res Treat. 2014 Feb;144(1):123-31.
Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, Ferrer-Lozano J, Perez-Fidalgo JA, Cristofanilli M, Gómez H, Arteaga CL, Giltnane J, Balko JM, Cronin MT, Jarosz M, Sun J, Hawryluk M, Lipson D, Otto G, Ross JS, Dvir A, Soussan-Gutman L, Wolf I, Rubinek T, Gilmore L, Schnitt S, Come SE, Pusztai L, Stephens P, Brown M, Miller VA.
Clin Cancer Res. 2014 Apr 1;20(7):1757-1767.
D538G mutation in estrogen receptor-α: A novel mechanism for acquired endocrine resistance in breast cancer. Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, Dvir A, Soussan-Gutman L, Jeselsohn R, Yelensky R, Brown M, Miller VA, Sarid D, Rizel S, Klein B, Rubinek T, Wolf I.
Cancer Res. 2013 Dec 1;73(23):6856-64.
Epigenetic silencing of the tumor suppressor klotho in human breast cancer. Rubinek T, Shulman M, Israeli S, Bose S, Avraham A, Zundelevich A, Evron E, Gal-Yam EN, Kaufman B, Wolf I.
Breast Cancer Res Treat. 2012 Jun;133(2):649-57.
KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I.
Clin Cancer Res. 2011 Jul 1;17(13):4254-66.
Functional variant of KLOTHO: a breast cancer risk modifier among BRCA1 mutation carriers of Ashkenazi origin. Wolf I, Laitman Y, Rubinek T, Abramovitz L, Novikov I, Beeri R, Kuro-O M, Koeffler HP, Catane R, Freedman LS, Levy-Lahad E, Karlan BY, Friedman E, Kaufman B.
Oncogene. 2010 Jan 7;29(1):26-33.
Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T.
Oncogene. 2008 Nov 27;27(56):7094-105.
The Longevity Hormone Klotho is a New Player in the Interacion of the Growth Hormone/Insulin-Like Growth Factor 1 Axis. Rubinek T, Wolf I, Modan-Moses D.
Pediatr Endocrinol Rev. 2016 Sep;14(1):9-18.
The Role of Alpha-Klotho as a Universal Tumor Suppressor. Rubinek T, Wolf I.
Vitam Horm. 2016;101:197-214.
Klotho and the Growth Hormone/Insulin-Like Growth Factor 1 Axis: Novel Insights into Complex Interactions. Rubinek T, Modan-Moses D.
Vitam Horm. 2016;101:85-118.
Endocrine resistance in breast cancer: focus on the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin signaling pathway. Hasson SP, Rubinek T, Ryvo L, Wolf I.
Breast Care (Basel). 2013 Aug;8(4):248-55.